21 CFR Part 11 compliance is essential for those in regulated industries. In this article we will walk through what 21 CFR Part 11 entails and how dataPARC meets the necessary requirements of compliance.
Unlock the full potential of big data with dataPARC’s tools and features.
In regulated industries, the integrity of data isn’t just a priority—it’s the law. For companies under FDA oversight, 21 CFR Part 11 compliance isn’t optional; it’s the backbone of trust in electronic records and signatures. Non-compliance can result in severe consequences, from hefty fines to product recalls. That’s a risk no one wants to take.
But here’s the challenge: how do you ensure your software solutions not only collect and store data but do so in a way that’s reliable, secure, and audit-ready? Enter dataPARC, a powerful data Historian and suite of tools designed to bring your process data into compliance with ALCOA+ principles—Attributable, Legible, Contemporaneous, Original, Accurate, and more.
With dataPARC, compliance becomes a proactive advantage, not a burden. We’re talking about robust user authentication, detailed audit trails, and data integrity practices that go beyond the basics. This isn’t just about ticking regulatory boxes; it’s about protecting your operations and your reputation.
Ready to dive into the specifics and discover how dataPARC can simplify compliance for your team? Let’s get started!
What is 21 CFR Part 11?
Think electronic records are easier to manage than paper ones? That’s true, until you need to prove those records are accurate, unaltered, and reliable. That’s where 21 CFR Part 11 comes in. Created by the FDA, this regulation ensures that electronic records and signatures meet the same rigorous standards as traditional paper documentation. Why? Because data integrity can make or break product quality, consumer safety, and your company’s compliance standing.
At its core, 21 CFR Part 11 sets the criteria for how electronic records and signatures must be managed. It’s not just about keeping records; it’s about keeping trustworthy records. This regulation ensures that electronic data is secure, traceable, and equivalent to paper records in every way that matters—accountability, reliability, and authenticity.
What does that look like in practice? Part 11 covers three main areas:
- Electronic Records – Your records must be accurate and complete. They need to be stored securely, easily retrievable, and protected against unauthorized changes.
- Electronic Signatures – Think of this as the digital version of a handwritten signature. These signatures must be unique to each user, verifiable, and tamper-proof.
- Audit Trails – Every action, from data creation to edits, must leave a clear trail. Who did it, when they did it, and what changed, all of it must be recorded.
Here’s the key takeaway: 21 CFR Part 11 isn’t just for legal compliance. It’s for operational excellence. Whether you’re producing pharmaceuticals, medical devices, or food, compliance ensures that every step of your process is documented, traceable, and defensible.
That’s a tall order, but it’s achievable with the right tools. With dataPARC, these standards aren’t just met, they’re built into every layer of the system. Let’s explore how ALCOA+ principles turn compliance into a practical, manageable part of your everyday operations.
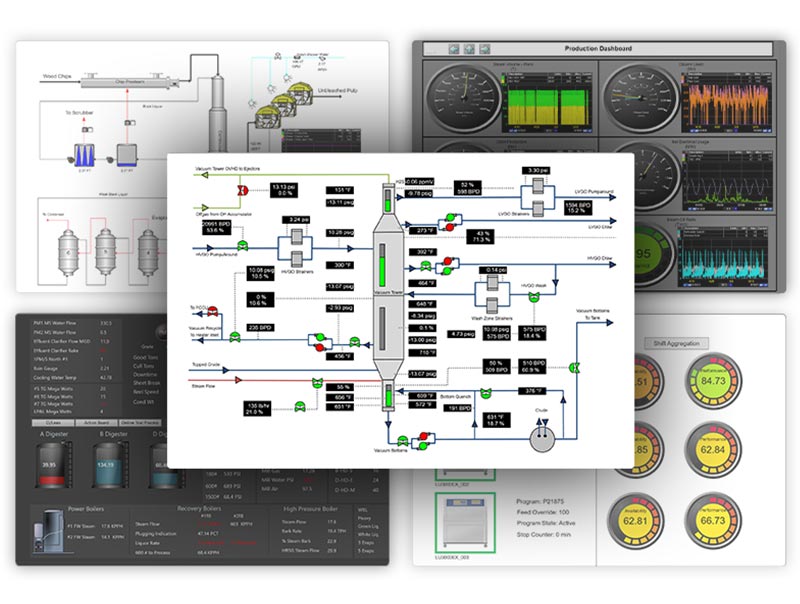
Check out dataPARC’s real-time process data analytics tools & see how better data access can help your business.
ALCOA+ Principles in 21 CFR Part 11 Compliance
“Good data is reliable data.” That’s the guiding principle behind ALCOA, the framework that ensures data integrity in regulated industries. Originally developed by the FDA, ALCOA lays out five core attributes that every piece of data must have to meet compliance standards: Attributable, Legible, Contemporaneous, Original, and Accurate. But in today’s digital world, those requirements have expanded into ALCOA+, adding Available, Enduring, Consistent, and Complete to the mix.
Why does this matter? Because without these attributes, your electronic records are just numbers on a screen, meaningless and unverifiable. In industries where lives and safety are on the line, data integrity isn’t negotiable. Let’s break down what these principles mean and how they apply to 21 CFR Part 11 compliance.
ALCOA: The Foundation of Data Integrity
- Attributable: Every action must be tied to a specific user. Who created the record? Who edited it? This ensures accountability at every step.
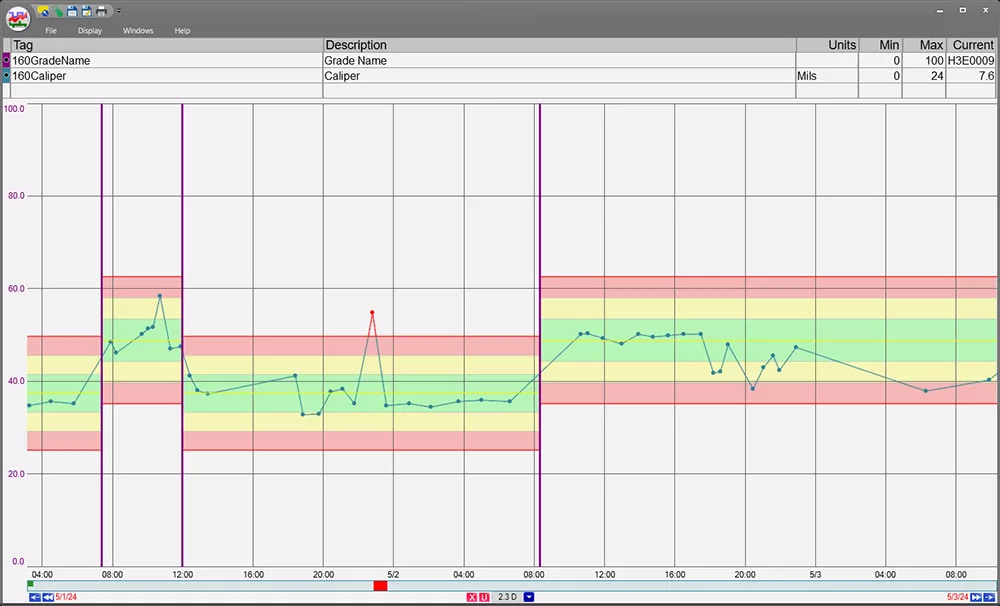
- Legible: Data must be clear, readable, and understandable. Confusing or corrupted records won’t pass an audit.
- Contemporaneous: Records must be created in real time. Delayed data entry introduces risks of errors and inaccuracies.
- Original: Source data is king. Copies or unverified entries can’t replace the original.
- Accurate: Precision is critical. Incomplete or incorrect data can lead to regulatory violations—or worse, unsafe products.
ALCOA+: The Modern Expansion
- Available: Data must be accessible when needed. Backup systems and reliable storage are non-negotiable.
- Enduring: Records must be preserved over time. This ensures historical data is intact for future audits or reviews.
- Consistent: Data should follow standardized processes. Variations can erode trust in its accuracy.
- Complete: Partial records aren’t enough. Every piece of data, from creation to deletion, must be captured.
How dataPARC Implements ALCOA+
With these principles in mind, dataPARC is built to deliver compliance seamlessly. For example:
- Attributable: User authentication features, such as Active Directory integration, ensure that every action is tied to a unique individual.
- Legible: Data visualization tools in dataPARC make even complex data sets easy to interpret.
- Contemporaneous: Real-time data collection ensures records are created as events happen.
- Available and Enduring: Backup configurations and dual-location storage keep your data secure and retrievable, even in the face of failures.
- Complete: Audit trails capture every detail, from edits to configuration changes, leaving no gaps in the record.
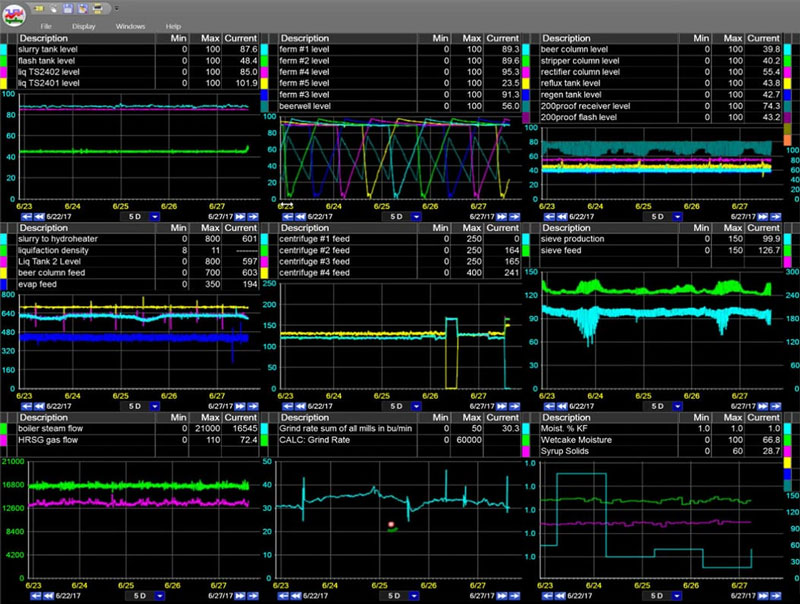
dataPARC not only allows for real-time data collection, but its intuitive trending package enables real-time production monitoring.
These principles aren’t just theoretical, they’re practical tools for ensuring compliance while optimizing your operations. ALCOA+ provides the framework, but dataPARC gives you the tools to make it actionable.
Now that we’ve covered the “why,” let’s dig into the “how.” In the next section, we’ll explore the features that make dataPARC a trusted partner for 21 CFR Part 11 compliance.
How dataPARC Tools and Historian Support Compliance
“Your data is only as secure as the systems protecting it.” In the world of 21 CFR Part 11 compliance, every layer of your process matters, from user authentication to audit trails. That’s where dataPARC shines. It’s designed to embed compliance into your workflows, ensuring that your records are not only accurate but also traceable, secure, and easy to manage. Let’s break down how dataPARC makes compliance practical and actionable.
User Access and Security
User account management is the cornerstone of any compliance strategy. With dataPARC, every user action is tied to a unique identity, ensuring accountability and security. The system offers three authentication methods: Anonymous Login, Username + Password, and Windows Integrated (Active Directory). While anonymous login has its uses, it’s not suitable for compliance. Instead, the Username + Password option provides strong security by enforcing unique credentials, password policies, and expirations. For the highest level of protection, the Windows Integrated method eliminates the need for credential entry, minimizing the risk of interception. Using these features strategically, such as disabling the “Public Option” for Active Directory logins, ensures compliance with ALCOA+ principles like Attributability and Originality.
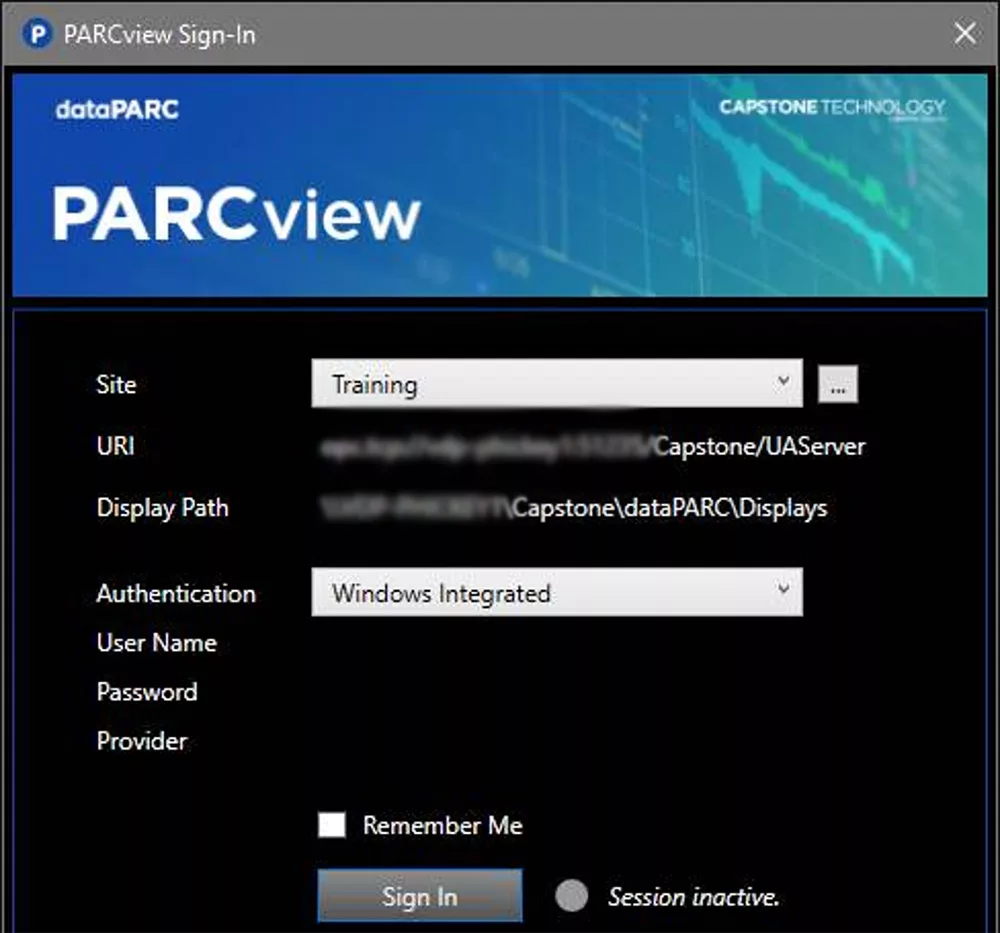
dataPARC allows for multiple security options including windows integrated.
Managing user access is just as critical. In dataPARC, permissions are assigned at a granular level, tailored to specific roles or groups. This role-based access control ensures that users only see and interact with the parts of the system they’re authorized to use. For example, operators might only monitor processes, while administrators manage configurations. Security profiles simplify this process by grouping permissions into manageable sets, which can be applied across teams or entire facilities. By creating a hierarchy of permissions, dataPARC reduces complexity and ensures consistent access control, aligning with the Consistency and Completeness principles of ALCOA+.
Data Integrity and Audit Trails
Data integrity is another area where dataPARC delivers exceptional value. The dataPARC Historian does not allow edits once a value has been written. Additionally, SQL databases form the backbone of data storage, and with dataPARC, you can enforce best practices like granting minimal necessary permissions and avoiding direct write access. Regular backups, stored in dual locations, ensure that your data remains Available and Enduring even during unexpected failures. Pairing this with dataPARC’s visualization tools allows you to access and analyze data in real-time, ensuring every decision is based on accurate and up-to-date information.
Audit trails are where compliance meets transparency. In dataPARC, every action—whether it’s a manual data entry, a configuration update, or a role change—is logged with a timestamp, user identity, and action details. These trails provide a clear record of events, ensuring Contemporaneousness and Attributability. Beyond the default audit capabilities, dataPARC supports customization for specific needs. For instance, you can set up custom tables for root cause analyses, like Five Why investigations, enabling deeper insights into operational challenges.
Compliance isn’t just about meeting regulatory standards; it’s about building systems that enhance trust and efficiency. dataPARC does this by embedding secure logins, role-based access, and transparent audit trails directly into your workflows. With these features, compliance becomes part of your everyday operations, not a separate task or burden. The result? A system that protects your data, streamlines your processes, and positions your business for long-term success. Ready to see how these features can work for you? Let’s dive deeper into the practical applications of compliance in the next section.
Practical Applications of Part 11 Compliance in dataPARC
Compliance isn’t just a checkbox—it’s a competitive edge. Industries regulated by the FDA, from pharmaceuticals to food and beverage, rely on tools like dataPARC to meet the rigorous demands of 21 CFR Part 11. But what does compliance actually look like in practice? Let’s explore how dataPARC’s features integrate seamlessly into real-world workflows to protect data integrity and ensure regulatory success.
Data Entry
Imagine managing a production line for a pharmaceutical product. Each step generates critical data, from temperature readings to batch logs. With dataPARC, every action is tied to a specific user through secure authentication. Whether an operator logs in to monitor a process or a manager signs off on a batch report, you know exactly who did what.
Changes to data, such as edits to manual entries, are logged in an audit trail that captures the timestamp, action, and user. This ensures Attributability and accuracy in every record. When inspectors review your processes, you can present clear, traceable records that demonstrate compliance with Part 11 requirements.
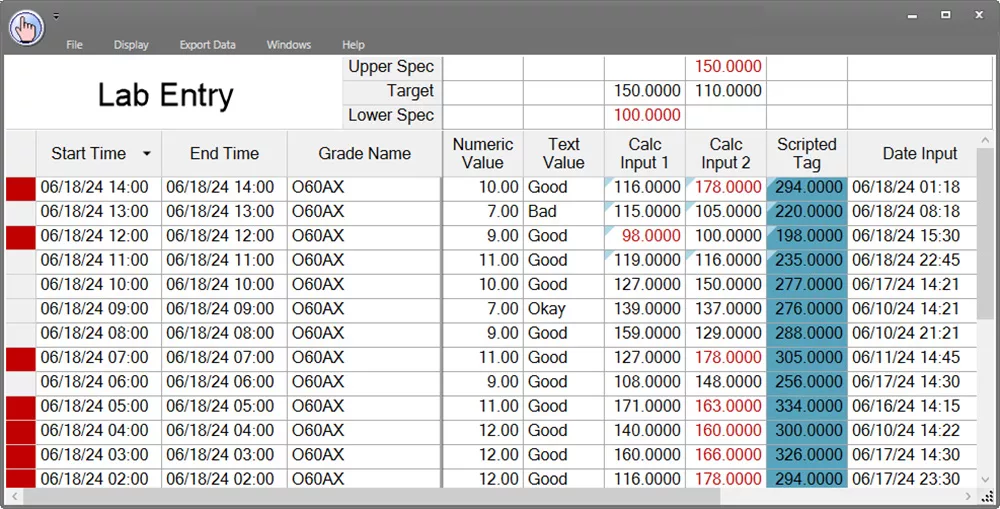
dataPARC’s Manual Data Entry display has a lot of compliance features including right click to view audits and the blue flag in the upper left corner of the cell indicates the value has been changed.
Reporting
Data reporting is often a headache during audits, but dataPARC simplifies the process. Predefined report templates allow you to quickly generate summaries that combine SQL data and manual entries. Reports can be exported in secure, read-only formats like PDFs, ensuring the data remains unaltered. For example, a food manufacturer can create a report that combines real-time sensor data with lab test results. This saves time, reduces errors, and gives inspectors confidence in the data’s integrity.
System Integration
Many facilities rely on multiple systems to monitor and control operations. dataPARC bridges the gap by pulling real-time data from external historians or control systems while maintaining the integrity of your records. With SQL backups and dual-location storage, data remains enduring and available, even if one system fails. This cross-system integration is a game-changer for facilities that rely on legacy systems, ensuring compliance without requiring a complete infrastructure overhaul.
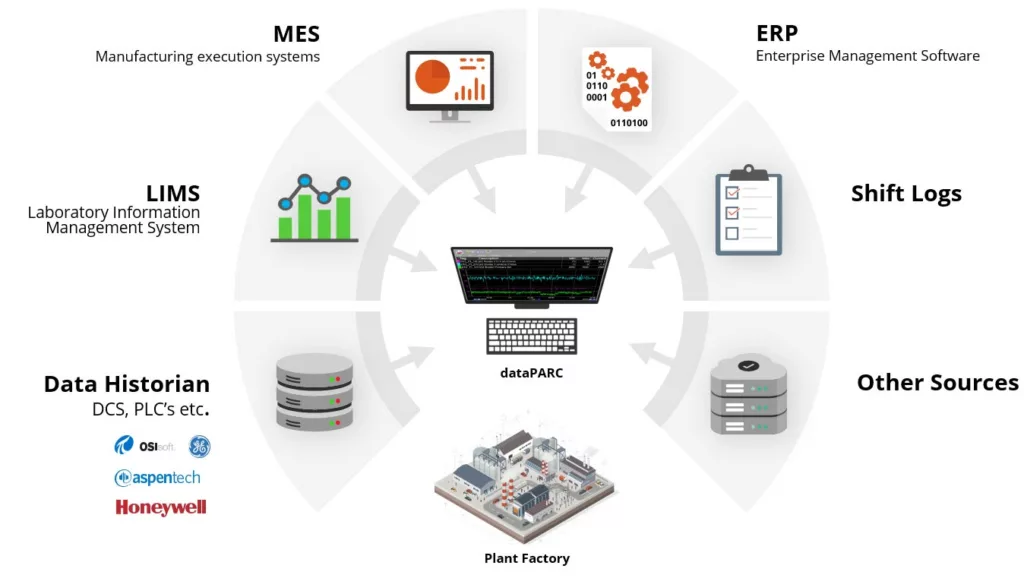
Have a single source of truth with your data by integrating with dataPARC. It acts as a manufacturing data integration platform pulling all your sources together to view in one place.
Real-Time Event Tracking
When something goes wrong, time is of the essence. With dataPARC’s alarming and customizable features, root cause analysis becomes faster and more effective. Custom tables for Five Why analysis link actions and events, helping you uncover root causes quickly. Audit trails for manual data entries and tag changes allow you to pinpoint when and where issues occurred. For instance, a beverage manufacturer can track a product defect to a specific batch and process adjustments, using the audit trail to identify and correct the error immediately.

Flexibility
21 CFR Part 11 isn’t static. Regulations evolve, and so should your systems. dataPARC’s flexible architecture ensures you can adapt seamlessly. Update user access settings as security best practices improve, or adjust audit trail configurations to meet new compliance requirements. By investing in a system that grows with your operations, you reduce risk while staying ahead of regulatory changes.
Compliance with 21 CFR Part 11 is about more than avoiding fines—it’s about protecting your brand, your customers, and your future. dataPARC gives you the tools to not only meet these standards but to integrate them into everyday operations, making compliance part of your workflow rather than a last-minute scramble.
So is the dataPARC 21 Historian and Suite CFR Part 11 Compliant?
“Compliance is the foundation of trust.” In industries regulated by the FDA, confidence begins with 21 CFR Part 11. This mandates that electronic records and signatures uphold the utmost levels of reliability, accuracy, and security. Reaching this level of compliance doesn’t need to be difficult – dataPARC ensures it is attainable, scalable, and efficient. So YES – the dataPARC Historian and Suite of Tools enables customers to meet 21 CFR Part 11 Compliance.
With secure user authentication, solid audit trails, and adaptable data management, dataPARC provides you with tools that exceed compliance. It fits effortlessly into your processes, guaranteeing that your records remain precise, verifiable, and prepared for audits, regardless of whether you’re in the pharmaceutical, food and beverage sectors or another regulated field. dataPARC transforms regulatory obligations into operational advantages.
The advantages extend beyond just complying with Part 11 standards. With dataPARC, you achieve improved efficiency in reporting, better decision-making abilities, and reassurance that your data is protected, safe, and lasting. It isn’t solely about steering clear of penalties or succeeding in audits; it’s about safeguarding your brand, engaging your procedures, and providing top-notch products for your clients.
Compliance with regulations is intricate, yet with appropriate tools, it can be a foundation for your success. dataPARC streamlines the procedure, allowing you to concentrate on innovation, expansion, and achieving outstanding results. Prepared to turn compliance into a strategic benefit? Reach out to us today to discover how dataPARC can benefit your business!

Learn more about dataPARC and Request a Demo Today!







